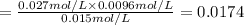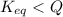At 1173 k, keq = 0.0108 for the following reaction: caco3(s) ⇄ cao(s) + co2(g) the reaction takes place in a 10.0 l vessel at 1173 k. if a mixture of 15.0 g caco3, 15.0 g cao, and 4.25 g co2 is allowed to approach equilibrium, what will happen to the amount of caco3? group of answer choices
- it will remain the same
- it will increase
- not enough information is provided to answer this question
- it will decrease

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, jusicca1109
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 03:30, ilizzy1224
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3
You know the right answer?
At 1173 k, keq = 0.0108 for the following reaction: caco3(s) ⇄ cao(s) + co2(g) the reaction takes p...
Questions in other subjects:


Geography, 11.10.2020 02:01


Biology, 11.10.2020 02:01

Chemistry, 11.10.2020 02:01


History, 11.10.2020 02:01

Mathematics, 11.10.2020 02:01

Computers and Technology, 11.10.2020 02:01

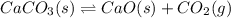
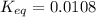
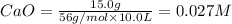
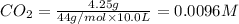
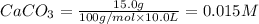
![Q=\frac{[CaO][CO_2]}{[CaCO_3]}](/tpl/images/0308/1118/76f7b.png)