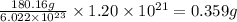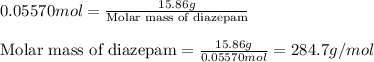Chemistry, 06.10.2019 07:01 mommytobe2019
What is the mass, in grams, of 1.50 mol of iron (iii) sulfate? express your answer using three significant figures. mm = nothing g request answer part b how many moles of ammonium ions are in 6.935 g of ammonium carbonate? express your answer using four significant figures. nn = nothing mol request answer part c what is the mass, in grams, of 1.20×1021 molecules of aspirin, c9h8o4? express your answer using three significant figures. mm = nothing g request answer part d what is the molar mass of diazepam (valium®) if 0.05570 mol weighs 15.86 g? express your answer using four significant figures. mm = nothing g/mol

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2

Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3

Chemistry, 22.06.2019 22:30, wpatskiteh7203
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
What is the mass, in grams, of 1.50 mol of iron (iii) sulfate? express your answer using three sign...
Questions in other subjects:










 .....(1)
.....(1)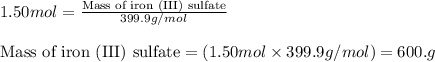
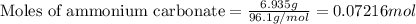

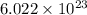 number of molecules occupies 1 mole
number of molecules occupies 1 mole