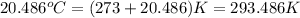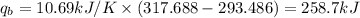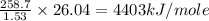Chemistry, 01.10.2019 17:20 mauifrifer3986
Oxyacetylene torches produce such high temperature that they are often used to weld and cut metal. when 1.53 g of acetylene (c2h2) is burned in a bomb calorimeter with a heat capacity of 10.69 kj/k, the temperature increases from 20.486°c to 44.688°c. what is δe (in kj/mol) for this combustion reaction? enter to 0 decimal places.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 01:00, jazzy200232
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 01:30, heavendl13
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1


Chemistry, 23.06.2019 08:30, elijah4723
If you had to research a particular disease or area of concern in veterinary medicine and science, which one would you choose? why?
Answers: 1
You know the right answer?
Oxyacetylene torches produce such high temperature that they are often used to weld and cut metal. w...
Questions in other subjects:


Geography, 26.02.2020 18:02







Physics, 26.02.2020 18:03

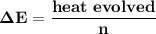
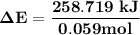
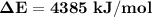
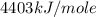
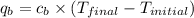
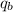 = heat absorbed by calorimeter = ?
= heat absorbed by calorimeter = ?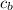 = specific heat of = 10.69 kJ/K
= specific heat of = 10.69 kJ/K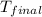 = final temperature =
= final temperature = 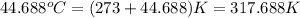
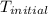 = initial temperature =
= initial temperature =