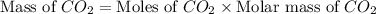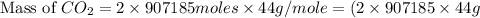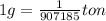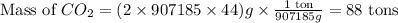Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, carlybeavers50
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1


You know the right answer?
How many tons of co2 are in 2 ton-moles of carbon dioxide? o 22 tons o 56 tons o 44 tons o 88 tons...
Questions in other subjects:


Mathematics, 05.11.2020 14:00

Mathematics, 05.11.2020 14:00

Mathematics, 05.11.2020 14:00

English, 05.11.2020 14:00

History, 05.11.2020 14:00



Mathematics, 05.11.2020 14:00

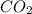 are 88 tons.
are 88 tons.