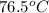Chemistry, 24.09.2019 20:00 andrecoral105
Amixture of 454 kg of applesauce at 10 degrees celsius is heated in a heat exchanger by adding 121300 kj. calculate the outlet temperature of the applesauce. (hint: heat capacity for applesauce is given at 32.8 degrees celsius. assume that this is constant and use this as the average.)

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1
You know the right answer?
Amixture of 454 kg of applesauce at 10 degrees celsius is heated in a heat exchanger by adding 12130...
Questions in other subjects:



Computers and Technology, 29.09.2019 01:10

Mathematics, 29.09.2019 01:10

Mathematics, 29.09.2019 01:10


Business, 29.09.2019 01:10

Mathematics, 29.09.2019 01:10


English, 29.09.2019 01:10

 ) of apple sauce at
) of apple sauce at  = 4.0177
= 4.0177 


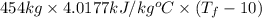
 =
=