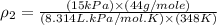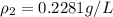Chemistry, 24.09.2019 19:00 saskiat1155
10 m3 of carbon dioxide is originally at a temperature of 50 °c and pressure of 10 kpa. determine the new density and volume of the carbon dioxide if the temperature and pressure change to 75 oc and 15 kpa.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

You know the right answer?
10 m3 of carbon dioxide is originally at a temperature of 50 °c and pressure of 10 kpa. determine th...
Questions in other subjects:










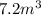 respectively.
respectively.
 = initial pressure of gas = 10 kPa
= initial pressure of gas = 10 kPa = final pressure of gas = 15 kPa
= final pressure of gas = 15 kPa = initial volume of gas =
= initial volume of gas = 
 = final volume of gas = ?
= final volume of gas = ? = initial temperature of gas =
= initial temperature of gas = 
 = final temperature of gas =
= final temperature of gas = 




 = new density
= new density