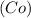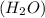Chemistry, 19.09.2019 03:30 cassandramanuel
while elements and compounds can both be classified as pure substances because they have distinct properties and composition, cannot be decomposed further.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, chloe081
We just started a new lesson in chemistry and everyone hates it and i dont get it one bit. i hate school. h el p. balanced equationc3h8+5o2-> 3co2+4h2o1.) if you start with 14.8g of propane(c3h8) and 3.44g of oxygen, which is the limiting reactant -check my answer 2.)what mass of excess reagent is left over? 3.)what mass of carbon dioxide can be made? 4.)what mass of water is produced?
Answers: 2

Chemistry, 21.06.2019 13:30, astultz309459
What is the molecular formula of a hydrocarbon with m+ = 166? (write the formula with no subscripts, e. g. c4h10.) what is the sum of rings and double bonds in this compound?
Answers: 1

Chemistry, 21.06.2019 22:30, granthazenp5e9mj
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

You know the right answer?
while elements and compounds can both be classified as pure substances because they have distinct pr...
Questions in other subjects:

Business, 13.07.2019 19:30

Geography, 13.07.2019 19:30

Social Studies, 13.07.2019 19:30

Mathematics, 13.07.2019 19:30

Chemistry, 13.07.2019 19:30


Mathematics, 13.07.2019 19:30