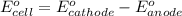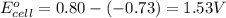Use the following half-reactions to construct a voltaic cell:
cr3+(aq) + 3e−\rightarrow→ cr(s...

Chemistry, 02.09.2019 19:20 bayleeharris8p78txa
Use the following half-reactions to construct a voltaic cell:
cr3+(aq) + 3e−\rightarrow→ cr(s) eo = −0.73 v
ag+(aq) + e−\rightarrow→ ag(s) eo = 0.80 v
determine the balanced overall redox reaction, and calculate eocell.
3 ag(s) + cr3+(aq) \rightarrow→ 3 ag+(aq) + cr(s), eocell = −1.53 v
3 ag+(aq) + cr(s) \rightarrow→ 3 ag(s) + cr3+(aq),eocell = +3.13 v
3 ag(s) + cr3+(aq) \rightarrow → 3 ag+(aq) + cr(s), eocell = −3.13 v
ag(s) + cr3+(aq) \rightarrow→ ag+(aq) + cr(s), eocell = −1.53 v
3 ag+(aq) + cr(s) \rightarrow→ 3 ag(s) + cr3+(aq), eocell = +1.53 v
ag+(aq) + cr(s) \rightarrow→ ag(s) + cr3+(aq), eocell = +1.53 v

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, aubreykenzie686
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 07:00, daniellekennedy05
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
Questions in other subjects:

History, 25.06.2019 08:30

Mathematics, 25.06.2019 08:30

Mathematics, 25.06.2019 08:30


Social Studies, 25.06.2019 08:30

Mathematics, 25.06.2019 08:30

Mathematics, 25.06.2019 08:30


Mathematics, 25.06.2019 08:30

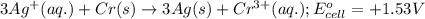
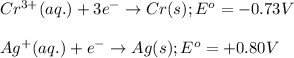
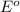 potential will always get reduced and will undergo reduction reaction. Here, silver will always undergo reduction reaction will get reduced.
potential will always get reduced and will undergo reduction reaction. Here, silver will always undergo reduction reaction will get reduced.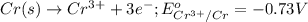
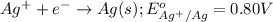 ( × 3)
( × 3)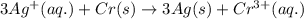
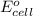 of the reaction, we use the equation:
of the reaction, we use the equation: