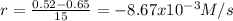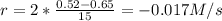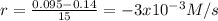a + b --> 2c

Chemistry, 30.08.2019 23:30 angsoccer02
Concentration time data were collected for the following reaction at 30 oc
a + b --> 2c
concentration time data at t = 30oc
time (s) [a] (mol l-1)
0 0.65
15 0.52
30 0.35
45 0.28
60 0.22
75 0.14
90 0.095
(i) calculate the average rate of consumption of a in the first 15 seconds of reaction.
(ii) calculate the average rate of production of c in the first 15 seconds of reaction.
(iii) calculate the average rate of consumption of a in the last 15 seconds of the reaction.
(iv) explain the difference between the rates of consumption calculated in (i) and that in (iii)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, tifftifftiff5069
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1
You know the right answer?
Concentration time data were collected for the following reaction at 30 oc
a + b --> 2c
a + b --> 2c
Questions in other subjects:

Chemistry, 13.03.2020 04:33





History, 13.03.2020 04:34




![r=\frac{\Delta [Concentration]}{\Delta t}](/tpl/images/0213/3224/c01cf.png)