
Ch4 and h2o were mixed in a 0.64 l reactor at 1800 k. steam reforming took place according to: ch4 (g) + h2o (9) co (g) + 3 h2 (9) the equilibrium constant for this reaction is k+0.28. at equilibrium, the reactor contained 0.36 mol of co, 0.081 mol of ha and 0.051 mol of ch. what is the concentration of h20 at equilibrium?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 14:30, lorrainelopez
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
Ch4 and h2o were mixed in a 0.64 l reactor at 1800 k. steam reforming took place according to: ch4...
Questions in other subjects:




Geography, 20.08.2019 16:10



Health, 20.08.2019 16:10

History, 20.08.2019 16:10

History, 20.08.2019 16:10

History, 20.08.2019 16:10

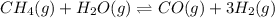

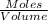
![[CO]=\frac{0.36 mol}{0.64 L}](/tpl/images/0189/2611/ede89.png)
![H_2=[H_2]=\frac{0.081 mol}{0.64 L}](/tpl/images/0189/2611/21859.png)
![CH_4=[CH_4]=\frac{0.051 mol}{0.64 L}](/tpl/images/0189/2611/7135e.png)
![H_2O=[H_2O]=?](/tpl/images/0189/2611/180f8.png)
![K_c=\frac{[CO][H_2]^3}{[CH_4][H_2O]}](/tpl/images/0189/2611/498b4.png)
![0.28=\frac{\frac{0.36 mol}{0.64 L}\times (\frac{0.081 mol}{0.64 L})^3}{\frac{0.051 mol}{0.64 L}\times [H_2O]}](/tpl/images/0189/2611/960b2.png)
![[H_2O]=0.05110 mol/L](/tpl/images/0189/2611/997c7.png)


