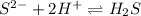Chemistry, 21.08.2019 05:30 emmareese2022
The best explanation for the dissolution of zns in dilute hcl is that a. the sulfide ion concentration is decreased by the formation of h2s. b. the zinc ion is amphoteric. c. the zinc ion concentration is decreased by the formation of a chloro complex. d. the sulfide ion concentration is decreased by oxidation to sulfur. e. the solubility product of zncl2 is less than that of zns.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, sammiehammer
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2



Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
The best explanation for the dissolution of zns in dilute hcl is that a. the sulfide ion concentrati...
Questions in other subjects:

Computers and Technology, 27.10.2020 17:40

Social Studies, 27.10.2020 17:40

Social Studies, 27.10.2020 17:40

Mathematics, 27.10.2020 17:40

Biology, 27.10.2020 17:40



Chemistry, 27.10.2020 17:40


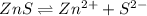
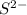 is a strong conjugate base of weak acid
is a strong conjugate base of weak acid 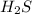 .
.