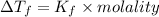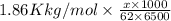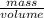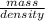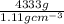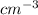Chemistry, 31.07.2019 20:30 shelbylynn17
Acommon antifreeze for car radiators is ethylene glycol, ch2(oh )ch2(oh ). how many millilite~s of this substance would you add to 6.5 l of water 1n the radiator if the coldest day in winter is - 20°c? would you keep this substance in the radiator in the summer to prevent the water from boiling? (the density and boiling point of ethylene glycol are 1.11 g cm^-3 and 470 k repsectively.)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 11:00, RidhaH
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural. question 2 reflects a moral or social value. question 3 refers to something that can be measured. question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 23:00, ceejay8005
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
Acommon antifreeze for car radiators is ethylene glycol, ch2(oh )ch2(oh ). how many millilite~s of t...
Questions in other subjects:


History, 31.01.2020 00:51

Mathematics, 31.01.2020 00:51


Chemistry, 31.01.2020 00:51



Geography, 31.01.2020 00:51


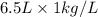 = 6.5 kg
= 6.5 kg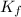 of water is 1.86 K kg/mol. And,
of water is 1.86 K kg/mol. And,