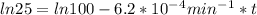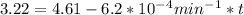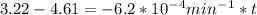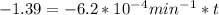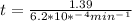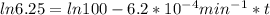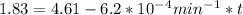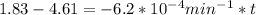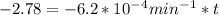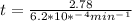Chemistry, 31.07.2019 20:30 YODIIZ6590
If the rate constant for the decomposition of n2o5 is 6.2 × 10−4/min, what is the half-life? (the rate law is first order in n2o5.) how long would it take for the concen- tration of n2o5 to decrease to 25% of its initial value? to 6.25% of its initial value?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, boonkgang6821
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2

Chemistry, 23.06.2019 18:30, cstevenson
Why must sample spots be above the solvent in paper chromatography
Answers: 1
You know the right answer?
If the rate constant for the decomposition of n2o5 is 6.2 × 10−4/min, what is the half-life? (the r...
Questions in other subjects:

Mathematics, 25.03.2021 14:20

Social Studies, 25.03.2021 14:20

Geography, 25.03.2021 14:20

Mathematics, 25.03.2021 14:20




English, 25.03.2021 14:20


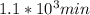 ,
, 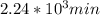 are taken for the concentration to decrease to 25% and
are taken for the concentration to decrease to 25% and 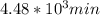 for the concentration to decrease to 6.25% .
for the concentration to decrease to 6.25% . 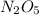 . For first order reaction, rate constant and half life are related to each other as:
. For first order reaction, rate constant and half life are related to each other as: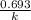
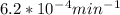
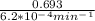
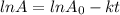
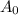 is initial and A is remaining concentration or amounts. k is rate constant and t is the time. Let's plug in the values and do calculations for t.
is initial and A is remaining concentration or amounts. k is rate constant and t is the time. Let's plug in the values and do calculations for t.