Chemistry, 26.06.2019 17:10 swelch2010
Consider the following reaction at a high temperature. br2(g) ⇆ 2br(g) when 1.35 moles of br2 are put in a 0.780−l flask, 3.60 percent of the br2 undergoes dissociation. calculate the equilibrium constant kc for the reaction.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:10, angellong94
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 09:30, lisbet123085
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 15:30, sanchez7489
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
Consider the following reaction at a high temperature. br2(g) ⇆ 2br(g) when 1.35 moles of br2 are pu...
Questions in other subjects:


Mathematics, 07.10.2019 12:30

Mathematics, 07.10.2019 12:30


Mathematics, 07.10.2019 12:30

Mathematics, 07.10.2019 12:30



Biology, 07.10.2019 12:30

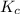 for the reaction is, 0.1133
for the reaction is, 0.1133 .
.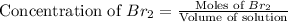
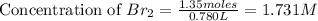
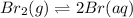
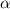 = 1.2 %
= 1.2 %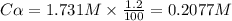
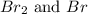 at equilibrium.
at equilibrium. = 2x = 2 × 0.2077 = 0.4154 M
= 2x = 2 × 0.2077 = 0.4154 M![K_c=\frac{[Br]^2}{[Br_2]}](/tpl/images/0020/2368/df6eb.png)