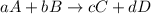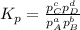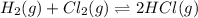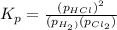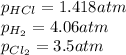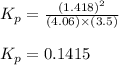Chemistry, 26.06.2019 03:20 elopezhilario6339
At a given temperature, 4.06 atm of h2 and 3.5 atm of cl2 are mixed and allowed to come to equilibrium. the equilibrium pressure of hcl is found to be 1.418 atm. calculate kp for the reaction at this temperature. h2(g) + cl2(g) < => 2 hcl(g)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, vivianni0727p1y30v
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2


Chemistry, 22.06.2019 15:30, vivianfling
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1
You know the right answer?
At a given temperature, 4.06 atm of h2 and 3.5 atm of cl2 are mixed and allowed to come to equilibri...
Questions in other subjects:










 for the given chemical reaction is 0.1415
for the given chemical reaction is 0.1415