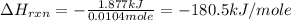Chemistry, 07.10.2019 16:20 chrismeldajbaptiste
You place 33.8 ml of 0.154 m ba(oh)2 in a coffee-cup calorimeter at 25.00°c and add 72.5 ml of 0.648 m hcl, also at 25.00°c. after stirring, the final temperature is 29.22°x. {assume that the total volume is the sum of the individual volumes and that the final solution has the same density (1.00 g/ml) and specific heat capacity (4.184 j/g°c) as water}. calculate the change in enthalpy, \deltaδh, of the reaction (in kj/mol) of water formed. enter the appropriate sign (+/ enter to 1 decimal place.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, livigrace9004
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 10:30, zayam1626
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:00, 1963038660
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1
You know the right answer?
You place 33.8 ml of 0.154 m ba(oh)2 in a coffee-cup calorimeter at 25.00°c and add 72.5 ml of 0.648...
Questions in other subjects:

Chemistry, 18.03.2021 01:10



Spanish, 18.03.2021 01:10

Biology, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10



Mathematics, 18.03.2021 01:10

English, 18.03.2021 01:10

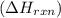 is, -180.5 kJ/mole
is, -180.5 kJ/mole
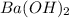
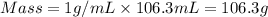
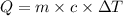
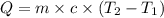
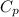 = specific heat capacity of water =
= specific heat capacity of water = 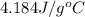
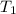 = initial temperature =
= initial temperature = 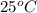
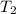 = final temperature =
= final temperature = 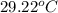
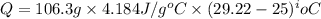
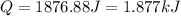 (1 kJ = 1000 J)
(1 kJ = 1000 J)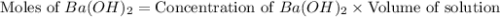
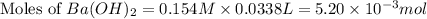
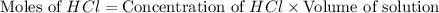
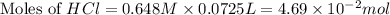
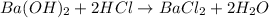
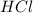
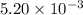 moles of
moles of 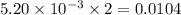 moles of
moles of 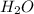
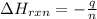
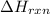 = enthalpy of reaction = ?
= enthalpy of reaction = ?