Chemistry, 03.02.2020 08:57 tasnimabdallah971
Energy and specific heat
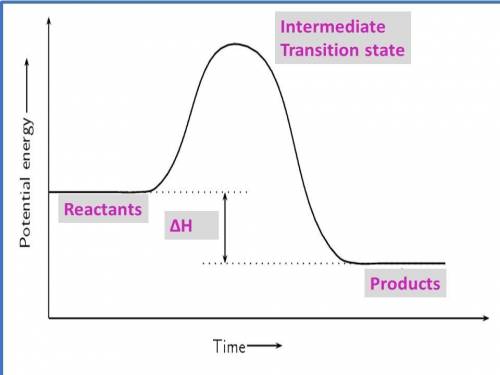
1. draw a graph of an exothermic reaction. label reactants, products and ∆h.
2. calculate the amount of energy required to raise the temperature of 3.00g of gold from 45.9 to 93.0°c.
3. 1.70g of a silvery metal requires 1000.j of energy to change its temp from 298k to 2749k. is the metal pure silver?


Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:10, mistiehaas
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 05:20, anggar20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1
You know the right answer?
Energy and specific heat
1. draw a graph of an exothermic reaction. label reactants, prod...
1. draw a graph of an exothermic reaction. label reactants, prod...
Questions in other subjects:

Biology, 16.04.2020 17:05

Mathematics, 16.04.2020 17:05


Mathematics, 16.04.2020 17:05