
Chemistry, 13.11.2019 00:31 zedanabdul198
Which statement can best be concluded from the ideal gas law? the product of pressure and volume of an ideal gas is proportional to the absolute temperature. all collisions between atoms or molecules are perfectly elastic and are not the result of any attractive forces. the temperature, pressure, and volume of a gas are all related. the behavior of a gas under real conditions does not obey the ideal gas law.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 23.06.2019 00:00, jasmin5285
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1


Chemistry, 23.06.2019 02:00, Robloxdemonduckyt
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2
You know the right answer?
Which statement can best be concluded from the ideal gas law? the product of pressure and volume of...
Questions in other subjects:


Mathematics, 12.02.2021 07:50

Business, 12.02.2021 07:50

Mathematics, 12.02.2021 07:50


Chemistry, 12.02.2021 07:50

Mathematics, 12.02.2021 07:50


Mathematics, 12.02.2021 07:50

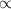 nT
nT


