

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, deidaraXneji
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Calculate the mass (g) of agcl formed when 174 g of silver sulfide reacts with excess hydrochloric a...
Questions in other subjects:



Mathematics, 06.12.2021 17:30

Social Studies, 06.12.2021 17:30

Mathematics, 06.12.2021 17:30

English, 06.12.2021 17:40

Geography, 06.12.2021 17:40

History, 06.12.2021 17:40


History, 06.12.2021 17:40

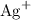 , andSulfide ions
, andSulfide ions 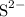 .
.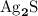 .
.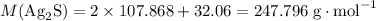 .
.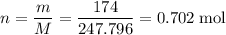 .
. .
.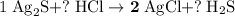 .
.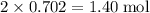 of AgCl.
of AgCl.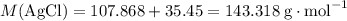 .
.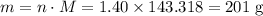 .
.

