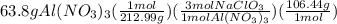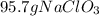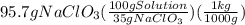Chemistry, 25.06.2019 18:30 carsondelane13
How many kilograms of a 35% m/m sodium chlorate solution is needed to react completely with 0.29 l of a 22% m/v aluminum nitrate solution

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, AbhiramAkella
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1


Chemistry, 22.06.2019 11:00, 21villalobosjabez
Which type of fossil does this image depict?
Answers: 1
You know the right answer?
How many kilograms of a 35% m/m sodium chlorate solution is needed to react completely with 0.29 l o...
Questions in other subjects:

Mathematics, 29.10.2021 09:30

Social Studies, 29.10.2021 09:30

English, 29.10.2021 09:30





Mathematics, 29.10.2021 09:40