
Chemistry, 30.06.2019 11:00 aaliyahnv07
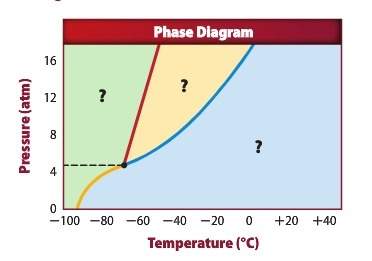
Using the diagram above answer the following questions: a) the green section of the graph represents: b) the yellow section of the graph represents: c) the blue section of the graph represents: d) what is the phase indicated by the yellow line if you are removing energy: e) at 12.00 atm and -40°c, what phase is present:

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, paulawells11
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 23:00, emilyphillips1681
If two identical atoms are bonded, what kind of molecule is formed
Answers: 1
You know the right answer?
Using the diagram above answer the following questions: a) the green section of the graph represen...
Questions in other subjects:

Mathematics, 03.12.2019 20:31

Mathematics, 03.12.2019 20:31

Mathematics, 03.12.2019 20:31

Mathematics, 03.12.2019 20:31


History, 03.12.2019 20:31






