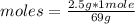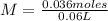Chemistry, 03.12.2019 20:31 michaellangley
What is the molarity of a solution prepared by dissolving 2.5 grams of lino3 in sufficient water to make 60.0 ml of solution?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:02, dondre54
Use the image below to answer the following questions there are three types of heat transfer occurring in the picture below. write a sentence about where each is occurring. (total three complete sentences in your own words.) on a very cold day, if you place your hand towards the bottom of a window, you can feel the convection of cold air as the cold, dense air sinks. try it out. did you feel the convection? it's a marshmallow on a metal stick over fire
Answers: 3


Chemistry, 22.06.2019 02:30, jrjimenez
At 40 âc the solution has at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. g of kno3 per 100 g of water and it can contain up to at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. g of kno3 per 100 g of water. at 0 âc the solubility is ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. kno3 per 100 g of water, so ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution. gkno3 per 100 g of water will precipitate out of solution. a kno3 solution containing 55 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 2

Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2
You know the right answer?
What is the molarity of a solution prepared by dissolving 2.5 grams of lino3 in sufficient water to...
Questions in other subjects:

Mathematics, 18.09.2020 19:01

Chemistry, 18.09.2020 19:01

Mathematics, 18.09.2020 19:01

Mathematics, 18.09.2020 19:01

Spanish, 18.09.2020 19:01

Mathematics, 18.09.2020 19:01

Mathematics, 18.09.2020 19:01

Mathematics, 18.09.2020 19:01

Mathematics, 18.09.2020 19:01

English, 18.09.2020 19:01

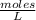
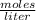 .
.