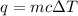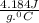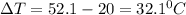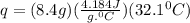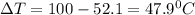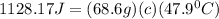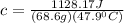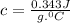A20 point reward for those who do a piece of unknown metal with mass 68.6 g is heated to an initial temperature of 100 °c and dropped into 8.4 g of water (with an initial temperature of 20 °c) in a calorimeter. the final temperature of the system is 52.1°c. the specific heat of water is 4.184 j/g*⁰c. what is the specific heat of the metal? a. 0.171 b. 0.343 c. 1.717 d. 3.433

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, girlwholikesanime
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 22.06.2019 02:30, dijonmckenzie3
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

You know the right answer?
A20 point reward for those who do a piece of unknown metal with mass 68.6 g is heated to an initia...
Questions in other subjects:




Biology, 05.05.2020 15:52




Mathematics, 05.05.2020 15:52

Mathematics, 05.05.2020 15:52

Health, 05.05.2020 15:52