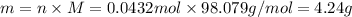Chemistry, 10.07.2019 03:30 hallmansean04
For each of the acid–base reactions, calculate the mass (in grams) of each acid necessary to completely react with and neutralize 4.85 g of the base. a. hcl(aq) + naoh(aq) s h2o(l) + nacl(aq) b. 2 hno3(aq) + ca(oh)2(aq) s 2 h2o(l) + ca(no3)2(aq) c. h2so4(aq) + 2 koh(aq) s 2 h2o(l) + k2so4(aq)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 04:50, mia36492
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table. state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2

Chemistry, 23.06.2019 10:20, Thejollyhellhound20
Based on the equation, how many grams of br2 are required to react completely with 29.2 grams of alcl3? alcl3 + br2 → albr3 + cl2 48.7 grams 52.6 grams 56.7 grams 61.3 grams
Answers: 3

You know the right answer?
For each of the acid–base reactions, calculate the mass (in grams) of each acid necessary to complet...
Questions in other subjects:


History, 17.12.2019 00:31

Mathematics, 17.12.2019 00:31



Computers and Technology, 17.12.2019 00:31


History, 17.12.2019 00:31

Social Studies, 17.12.2019 00:31

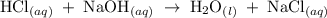
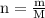
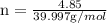
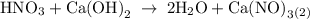
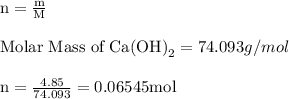
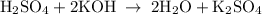

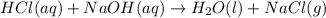
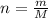
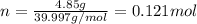
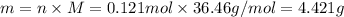
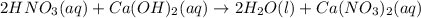
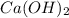 completely reacts with 2 mol of
completely reacts with 2 mol of 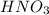 . The mass of
. The mass of 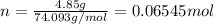
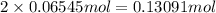 of
of 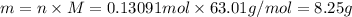
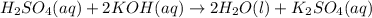
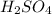 . The mass of KOH is given 4.85 g, convert this into number of moles as follows:
. The mass of KOH is given 4.85 g, convert this into number of moles as follows: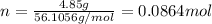
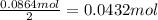 of
of