Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, gatorr2010
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3


Chemistry, 23.06.2019 00:30, danielmartinez024m
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1

Chemistry, 23.06.2019 01:30, koggebless
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
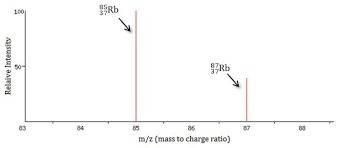
Rubidium has two naturally occurring isotopes with masses 84.91 amu and 86.91 amu and has an atomic...
Questions in other subjects:



English, 25.09.2020 02:01

History, 25.09.2020 02:01

Chemistry, 25.09.2020 02:01




Biology, 25.09.2020 02:01