
Chemistry, 12.07.2019 18:00 ethan62211
What is the difference between a suspension, a colloid, a solution, and an emulsion?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, kristieroth1
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 05:30, greekfreekisdbz
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
What is the difference between a suspension, a colloid, a solution, and an emulsion?...
Questions in other subjects:


Social Studies, 24.09.2019 04:00




Mathematics, 24.09.2019 04:00

Biology, 24.09.2019 04:00


Business, 24.09.2019 04:00

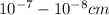 i.e. molecule in size. There is no effect of light occurs in the solution and solution can't filtered but can separated by the physical technique i.e. distillation.
i.e. molecule in size. There is no effect of light occurs in the solution and solution can't filtered but can separated by the physical technique i.e. distillation.

