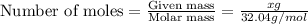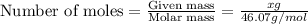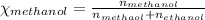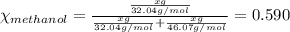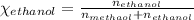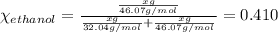Chemistry, 24.09.2019 04:00 abemorales
Asolution is made by mixing equal masses of methanol, ch4o, and ethanol, c2h6o. determine the mole fraction of each component to at least three signa solution is made by mixing equal masses of methanol, ch4o, and ethanol, c2h6o. determine the mole fraction of each component to at least three significant figures. ificant figures.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, nikejose11
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3



Chemistry, 23.06.2019 00:00, jasmin5285
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
Asolution is made by mixing equal masses of methanol, ch4o, and ethanol, c2h6o. determine the mole f...
Questions in other subjects:





History, 16.07.2019 04:30



Social Studies, 16.07.2019 04:30


Mathematics, 16.07.2019 04:30

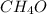 and ethanol
and ethanol 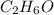 are mixed.
are mixed.