

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
You know the right answer?
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb2+ + 2cl -, the concentration of the prod...
Questions in other subjects:



Mathematics, 12.02.2020 03:12


Geography, 12.02.2020 03:12





Mathematics, 12.02.2020 03:12

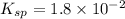
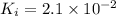
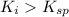 Precipitation
Precipitation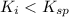 No Precipitation
No Precipitation

