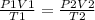Abubble of helium gas has a volume of 0.650 ml near the bottom of a large aquarium where the pressure is 1.54 atm and the temperature is 12°c. determine the bubble's volume upon rising near the top where the pressure is 1.01 atm and 16°c. assume that the number of moles of helium remains constant and that the helium is an ideal gas.

Answers: 1


Other questions on the subject: Physics



You know the right answer?
Abubble of helium gas has a volume of 0.650 ml near the bottom of a large aquarium where the pressur...
Questions in other subjects:

Mathematics, 19.05.2020 15:00


Mathematics, 19.05.2020 15:00




Biology, 19.05.2020 15:00

English, 19.05.2020 15:00

Mathematics, 19.05.2020 15:00

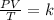 for a fixed amount of gas
for a fixed amount of gas