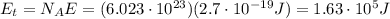Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 00:10, oktacos
The energy released by a chemical reaction can be measured using a calorimeter. when barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00 g of water in the calorimeter decreases from 30.0°c to 8.0°c. the equation for calculating energy absorbed or released by a reaction is: where q is the energy released or absorbed, m is the mass of water in the calorimeter, cp is the specific heat of water, and δt is the observed temperature change. if the specific heat of liquid water is 4.19 j/g·°c, how much energy was absorbed by the reaction?
Answers: 3

Physics, 22.06.2019 04:30, MadisonUpky9652
Which of the following are not typically included in the periodic table? a. atomic mass b. element symbol c. isotopes d. number of electrons
Answers: 2

Physics, 22.06.2019 16:30, safiyyahrahman6907
One number is said to be an "order of magnitude" larger than another number if choose one: a. it is 10 times larger. b. it is 5 times larger. c. it is 3 times larger. d. it is 100 times larger. e. it is 2 times larger.
Answers: 1

Physics, 22.06.2019 19:30, dragador7601
Which of the following compounds would be primarily ionic? methane, ch4, ammonia nh3, calcium chloride, cacl2, or carbon dioxide, co2
Answers: 1
You know the right answer?
How much energy (in j) is contained in 1.00 mole of 739 −nm photons?...
Questions in other subjects:



Mathematics, 21.05.2021 19:00

Biology, 21.05.2021 19:00

Chemistry, 21.05.2021 19:00

Mathematics, 21.05.2021 19:00




Mathematics, 21.05.2021 19:00

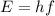
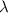 :
: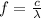
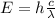
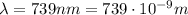 , so their energy is
, so their energy is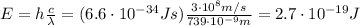
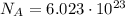 photons (Avogadro number)
photons (Avogadro number)