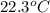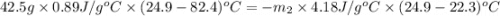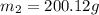Physics, 29.07.2019 02:00 christabell0303
A42.5 g piece of aluminum (which has a molar heat capacity of 24.03 j/ocmol) is heated to 82.4oc and dropped into a calorimeter containing water (specific heat capacity of water is 4.18 j/goc) initially at 22.3oc. the final temperature of the water is 24.9oc. calculate the mass of water in the calorimeter. ignore significant figures for this problem.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 08:30, bollipard1183
Brutus, the dog, is pulling a bone to the left with a force of 20 n. lassie, another dog, is pulling a bone to the right with a force of 18n. what is the net force? a. b. c.
Answers: 1

Physics, 22.06.2019 09:00, montgomeryaevans
This is really important 1.which of the following prefixes represents the largest value? (2 points)gigahectorkilomilli2.which of the following types of graphs is best for plotting the mean, median, and mode of data? (2 points)bar graphbox-and-whiskercircle graphstem-and-leaf
Answers: 1

Physics, 22.06.2019 09:30, abdominguez7187
An electric clothes dryer has a resistance of 8 ohms. it draws 30 a of current. what is the voltage, in volts, of the wall outlet that it is plugged into?
Answers: 2

Physics, 22.06.2019 15:30, tiffuuu
At 20∘c, the hole in an aluminum ring is 2.800 cm in diameter. you need to slip this ring over a steel shaft that has a room-temperature diameter of 2.804 cm . 1. to what common temperature should the ring and the shaft be heated so that the ring will just fit onto the shaft? coefficients of linear thermal expansion of steel and aluminum are 12×10−6 k−1 and23×10−6 k−1 respectively.
Answers: 1
You know the right answer?
A42.5 g piece of aluminum (which has a molar heat capacity of 24.03 j/ocmol) is heated to 82.4oc and...
Questions in other subjects:

Mathematics, 14.05.2021 19:50

Mathematics, 14.05.2021 19:50

Mathematics, 14.05.2021 19:50






Arts, 14.05.2021 19:50

Biology, 14.05.2021 19:50

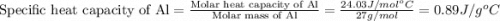
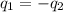
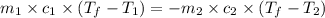
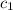 = specific heat of aluminum =
= specific heat of aluminum = 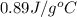
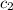 = specific heat of water =
= specific heat of water = 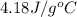
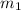 = mass of Al = 42.5 g
= mass of Al = 42.5 g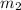 = mass of water = ?
= mass of water = ?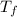 = final temperature of water =
= final temperature of water = 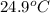
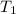 = initial temperature of Al =
= initial temperature of Al = 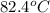
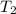 = initial temperature of water =
= initial temperature of water =