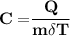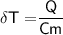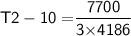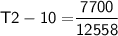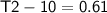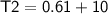Physics, 15.03.2022 01:00 nancyrj3860
3 kg of water (c = 4,186 J /kg°C) is at an initial temperature of 10°C. If 7,700 J of heat is applied, what will be its final temperature? Show all of your work and use the correct units for full credit.

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 05:00, damientran
The image below shows numerous volcanic mountains in the pacific northwest. what is the most likely cause of the volcanic and earthquake activity in this region?
Answers: 2



Physics, 23.06.2019 02:40, HannaTheGurls
If a rock is thrown upward on the planet mars with a velocity of 10 mys, its height in meters t seconds later is given by y − 10t 2 1.86t 2 . (a) find the average velocity over the given time intervals: (i) [1, 2] (ii) [1, 1.5] (iii) [1, 1.1] (iv) [1, 1.01] (v) [1, 1.001] (b) estimate the instantaneous velocity when t − 1.
Answers: 2
You know the right answer?
3 kg of water (c = 4,186 J /kg°C) is at an initial temperature of 10°C. If 7,700 J of heat is applie...
Questions in other subjects:


Mathematics, 26.03.2021 16:50


Biology, 26.03.2021 16:50





Mathematics, 26.03.2021 16:50

Mathematics, 26.03.2021 16:50