Can someone explain this pls ?
...

Physics, 24.01.2022 14:00 kokilavani
Can someone explain this pls ?
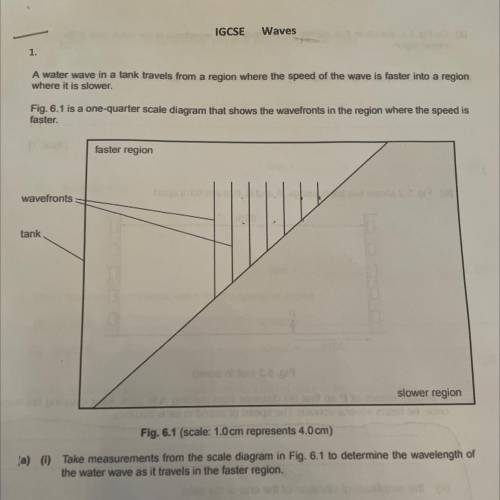

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 00:00, tsupreme
1134567 a jet with mass m = 1.1 ă— 105 kg jet accelerates down the runway for takeoff at 2 m/s2. 1) what is the net horizontal force on the airplane as it accelerates for takeoff? 2.2*10^5 n your submissions: 2.2*10^5 computed value: 220000submitted: saturday, january 26 at 2: 41 pm feedback: correct! 2) what is the net vertical force on the airplane as it accelerates for takeoff? 0 n your submissions: 0 computed value: 0submitted: saturday, january 26 at 2: 41 pm feedback: correct! 3) once off the ground, the plane climbs upward for 20 seconds. during this time, the vertical speed increases from zero to 21 m/s, while the horizontal speed increases from 80 m/s to 95 m/s. what is the net horizontal force on the airplane as it climbs upward? n 4) what is the net vertical force on the airplane as it climbs upward? n 5) after reaching cruising altitude, the plane levels off, keeping the horizontal speed constant, but smoothly reducing the vertical speed to zero, in 13 seconds. what is the net horizontal force on the airplane as it levels off? n 6) what is the net vertical force on the airplane as it levels off?
Answers: 1

Physics, 22.06.2019 14:10, chuchi24
The number of passengers who arrive at the platform of a subway station for the 10 am train is a random variable with a mean of 120 and a variance of 16. find the lower bound of the probability that there will be between 100 and 140 passengers (round off to second decimal place).
Answers: 3

Physics, 22.06.2019 16:30, taliajohnsom9901
Humidity is to blame for that muggy, steamy feeling you experience on some hot summer days. what gas in the atmosphere causes humidity? a) oxygen b) hydrogen c) nitrogen d) water vapor
Answers: 1

Physics, 22.06.2019 18:30, CaptainKiller528
In the united states, tornadoes generally occur because of the freezing of ocean water underwater earthquakes meeting of cool and warm air masses shifting of warm and cool ocean currents
Answers: 1
You know the right answer?
Questions in other subjects:

Health, 17.04.2021 21:40

Mathematics, 17.04.2021 21:40

Mathematics, 17.04.2021 21:40

History, 17.04.2021 21:40

Mathematics, 17.04.2021 21:40

Computers and Technology, 17.04.2021 21:40

Mathematics, 17.04.2021 21:40


Mathematics, 17.04.2021 21:40

Mathematics, 17.04.2021 21:40



