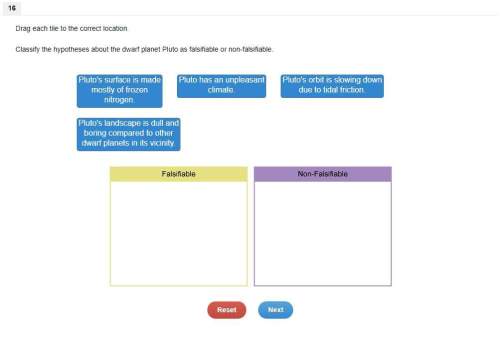
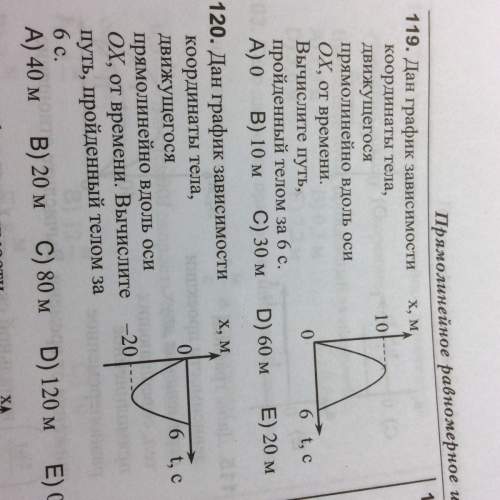Answers: 3


Other questions on the subject: Physics

Physics, 21.06.2019 16:30, jroy1973
In this experiment, you will use a track, a toy car, and some washers to explore newton’s first two laws of motion. you will make observations and collect data regarding the motion of these objects. in the space below, write a general scientific question that you will answer by doing this experiment.
Answers: 1

Physics, 21.06.2019 21:30, ddoherty88
Aquantity of gas has a volume of 1.5 m3 and an absolute pressure of 95 kpa. when the gas is compressed to a volume of 0.5 m3, what is the new absolute pressure of the gas? (assume that there’s no change in temperature.)
Answers: 3

Physics, 21.06.2019 23:50, diana156
Select the correct answer from each drop-down menu. compared to its surroundings, the concentration of solutes is low inside a cell. so, the cell is with respect to its surroundings. a particular solute in this cell uses energy for its transport from the cell to its surroundings. this type of transport is called
Answers: 3

Physics, 22.06.2019 04:00, idontknow11223344
Simple machines can a. increase force only b. decrease force only c. increase or decrease force
Answers: 3
You know the right answer?
A calorimeter has a heat capacity of 60J/°C. 0.15kg of water at 20°C is contained in the calorimeter...
Questions in other subjects:

Health, 23.10.2020 01:01

History, 23.10.2020 01:01

Medicine, 23.10.2020 01:01

Geography, 23.10.2020 01:01

Mathematics, 23.10.2020 01:01


Mathematics, 23.10.2020 01:01



Mathematics, 23.10.2020 01:01