
Physics, 13.07.2021 15:10 jayjay2006
A total of 25.6 kJ of heat energy is added to a 5.46 L sample of helium at 0.991 atm. The gas is allowed to expand against a fixed external pressure to a volume of 18.7 L .
Required:
a. Calculate the work done on or by the helium gas units of joules, J.
b. What is the change in the helium a internal energy in units kilojoules, KJ?

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 02:30, liltay12386
Which is an example of gaining a static charge by conduction? a) rubbing a balloon against your hair. b) shuffling your shoes across a carpet. c) bringing a charged rod near an electroscope. d) touching your car on a cold day and getting a shock.
Answers: 1


Physics, 22.06.2019 04:30, jakiyahporter0817
Asystem containing an ideal gas at a constant pressure of 1.22×10^5 pa gains 2140 j of heat. during the process, the internal energy of the system increases by 2320 j. what is the change in volume of the gas?
Answers: 3

Physics, 22.06.2019 04:50, andrewsaul04
The position of a crate sliding down a ramp is given by x=(0.05t^3) m, y=(1.7t^2) m, z=(6−0.85t^5/2) m, where t is in seconds. (a) determine the magnitude of the crate's velocity when t = 2 s. (b) determine the magnitude of the crate's acceleration when t = 2 s.
Answers: 2
You know the right answer?
A total of 25.6 kJ of heat energy is added to a 5.46 L sample of helium at 0.991 atm. The gas is all...
Questions in other subjects:


Mathematics, 11.02.2021 14:50





Mathematics, 11.02.2021 15:00

Mathematics, 11.02.2021 15:00

Mathematics, 11.02.2021 15:00

Mathematics, 11.02.2021 15:00

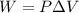
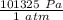 ) = 100413 Pa
) = 100413 Pa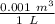 ) = 0.01324 m³
) = 0.01324 m³

