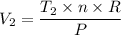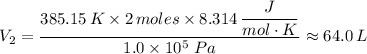Physics, 11.07.2021 18:00 puppylover72
When 2 moles of helium gas expand at a constant pressure p= 1.0×10^5 pascals, the temperature increase from 2 °c to 112 °c. If the initial volume of the gas was 45 liters. Cp= 20.8j/mol. K, Cv= 12.6j/mol. K. Determine i. The work done W by the gas as it exands

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 14:30, mangowammy
What conclusion can be made based on the temperature of soil when the light hits the soil at 0°, 45°, and 90° angles in section 2 of the experiment? did your results support your hypothesis? why or why not?
Answers: 1

Physics, 22.06.2019 15:30, tiffuuu
At 20∘c, the hole in an aluminum ring is 2.800 cm in diameter. you need to slip this ring over a steel shaft that has a room-temperature diameter of 2.804 cm . 1. to what common temperature should the ring and the shaft be heated so that the ring will just fit onto the shaft? coefficients of linear thermal expansion of steel and aluminum are 12×10−6 k−1 and23×10−6 k−1 respectively.
Answers: 1

Physics, 23.06.2019 00:30, briseisr20
3. which is not a primary color of light? blue red green yellow
Answers: 1

Physics, 23.06.2019 00:30, gamallopatty
What is the coldest temperature ever recorded in san antonio?
Answers: 1
You know the right answer?
When 2 moles of helium gas expand at a constant pressure p= 1.0×10^5 pascals, the temperature increa...
Questions in other subjects:

Spanish, 05.01.2020 16:31





Mathematics, 05.01.2020 16:31


Mathematics, 05.01.2020 16:31


Mathematics, 05.01.2020 16:31