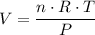. When 2 moles of helium gas expand at constant pressure P = 1.0 × 105 Pascals, the temperature increases from 2℃ to 112℃. If the initial volume of the gas was 45 liters. Cp=20.8J/mol. K, Cv=12.6J/mol. K. Determine i. The work done W by the gas as it expands (4) ii. The total heat applied to the gas (2) iii. The change in internal energy (2

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 02:00, ummsumaiyah4185
Write the correct abbreviation for each metric unit
Answers: 1

Physics, 22.06.2019 10:00, issaaamiaaa15
Which is the correct symbol for an isotope of iodine with 53 protons and 78 neutrons?
Answers: 2

You know the right answer?
. When 2 moles of helium gas expand at constant pressure P = 1.0 × 105 Pascals, the temperature incr...
Questions in other subjects:





Mathematics, 17.03.2020 21:00





Mathematics, 17.03.2020 21:00