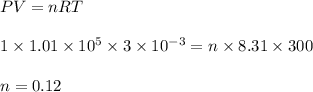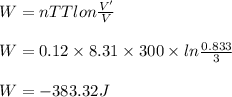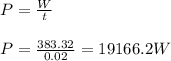Physics, 09.07.2021 22:20 wiredq2049
A cylinder of volume 3 liter has Argon gas initially at 300 K, and 1.00 atm pressure. The piston compresses the gas to a new pressure of 3.60 atm. During this compression, the temperature is maintained constant by using an appropriate heat sink. Find (a) the final volume of the gas, (b) the work done on the gas by the piston, (c) the energy transferred out by heat.(d) If the process takes 20 milliseconds, what is the power

Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 05:10, lololololol12555
What do elements in a family tend to share. a.) similar periods b.) similar groups c.) similar atomic symbols d.) similar chemical properties and characteristics
Answers: 2

Physics, 22.06.2019 06:00, ahmedeldyame
Why don't you see your reflection in water with waves or repples
Answers: 1

Physics, 22.06.2019 18:30, TheSpeedster
Two trains leave a station at the same time, train a travels at a constant speed of 16 m/s. train b starts at 8.0 m/s but accelerates constantly at 1.0 m/s squared. after 10.0 seconds, which train has the greater speed?
Answers: 1

Physics, 23.06.2019 01:30, gameranonymous266
The flame produced by the burner of a gas (propane) grill is a pale blue color when enough air mixes with the propane (c3h8) to burn it completely. for every gram of propane that flows through the burner, what volume of air is needed to burn it completely? assume that the temperature of the burner is 195.0°c, the pressure is 1.05 atm, and the mole fraction of o2 in air is 0.210.
Answers: 2
You know the right answer?
A cylinder of volume 3 liter has Argon gas initially at 300 K, and 1.00 atm pressure. The piston com...
Questions in other subjects:






Mathematics, 01.12.2020 16:20



Mathematics, 01.12.2020 16:20