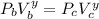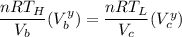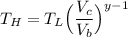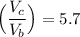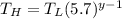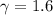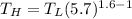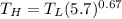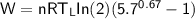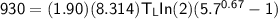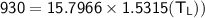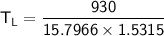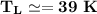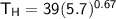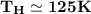The working substance of a certain Carnot engine is 1.90 of an ideal
monatomic gas. During the isothermal expansion portion of this engine's
cycle, the volume of the gas doubles, while during the adiabatic expansion
the volume increases by a factor of 5.7. The work output of the engine is
930 in each cycle.
Compute the temperatures of the two reservoirs between which this engine
operates.

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 18:00, justhereforanswers13
According to the law of conservation of mass, in a chemical reaction the total starting mass of all the reactants equal the total final mass of all the products. true or false?
Answers: 1

Physics, 22.06.2019 07:30, darkskinnednune
Write the function getkthdigit(n, k) that takes a possibly-negative int n and a non-negative int k, and returns the kth digit of n, starting from 0, counting from the right
Answers: 3

Physics, 22.06.2019 11:50, azertyqwerty123
Two resistors r1 and r2 may be connected either in series or parallel across an ideal battery with emf ε. we desire the rate of energy dissipation of the parallel combination to be 8.75 times that of the series combination. if r1 = 105 ω, what are the (a) smaller and (b) larger of the two values of r2 that result in that dissipation rate?
Answers: 2

Physics, 22.06.2019 13:10, alejandramirand9836
A0.750 kg aluminum pan is removed from the stove and plunged into a sink filled with 10.0 kg of water at 293 k. the water temperature quickly rises to 297 k. what was the initial temperature of the aluminum pan? the specific heat of aluminum is ca = 900 j/(kgk) and water is cw = 4190 j/(kgk)
Answers: 3
You know the right answer?
The working substance of a certain Carnot engine is 1.90 of an ideal
monatomic gas. During the isot...
Questions in other subjects:




Mathematics, 19.01.2020 00:31






 --- (1)
--- (1)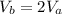
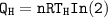
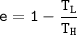
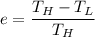
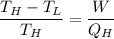
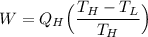
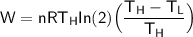
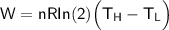
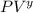 = constant
= constant