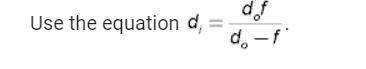An ice chest at a beach party contains 12 cans of soda at 3.78 °C. Each can of soda has a mass of 0.35 kg and a specific heat capacity of 3800 J/(kg C°). Someone adds a 6.48-kg watermelon at 29.4 °C to the chest. The specific heat capacity of watermelon is nearly the same as that of water. Ignore the specific heat capacity of the chest and determine the final temperature T of the soda and watermelon in degrees Celsius.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 11:00, stankyweezle
A0.580-kg rock is tied to the end of a string and is swung in a circle with a radius of 0.500 meters. the velocity of the rock is 4.50 m/s. what is the centripetal force acting on the rock? 15.5 n 5.22 n 69.8 n 23.5 n
Answers: 3



Physics, 23.06.2019 01:30, martinezkimberly706
Match each type of lever with the correct diagram. 1. first-class 2. third-class 3. second-class
Answers: 3
You know the right answer?
An ice chest at a beach party contains 12 cans of soda at 3.78 °C. Each can of soda has a mass of 0....
Questions in other subjects:



Mathematics, 20.01.2021 05:50



English, 20.01.2021 05:50

Chemistry, 20.01.2021 05:50


Mathematics, 20.01.2021 05:50

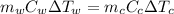
 = mass of watermelon = 6.48 kg
= mass of watermelon = 6.48 kg = mass of cans = (12)(0.35 kg) = 4.2 kg
= mass of cans = (12)(0.35 kg) = 4.2 kg = specific heat capacity of watermelon = 3800 J/kg.°C
= specific heat capacity of watermelon = 3800 J/kg.°C = specific heat capacity of cans = 4200 J/kg.°C
= specific heat capacity of cans = 4200 J/kg.°C = Change in Temprature of watermelon = 29.4°C - T
= Change in Temprature of watermelon = 29.4°C - T = Change in Temperature of cans = T - 3.78°C
= Change in Temperature of cans = T - 3.78°C