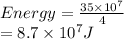The average speed of molecules of a 0.1 mole nitrogen gas in a container is 5103
m/s.
a/ Determine the total translational kinetic energy of the gas.
b/ Compute the energy as heat providing to the gas so that the average speed of its molecules increases to
double.

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 13:40, hannahgracew12
The de broglie relation λ=h/p can be rewritten in terms of the wave number k as p=kℏ. recall that wave number is defined by k=2π/λ. using the fact that λ=v/f, find the wave numbers k1 and k2 corresponding to frequencies f1 and f2. express your answer as two expressions
Answers: 2

Physics, 21.06.2019 22:30, celibe9391
What is the earliest point in the universe that we can observe? why can’t we see back further than this?
Answers: 2

You know the right answer?
The average speed of molecules of a 0.1 mole nitrogen gas in a container is 5103
m/s.
a/ D...
a/ D...
Questions in other subjects:


SAT, 25.12.2021 06:50


Mathematics, 25.12.2021 06:50

Advanced Placement (AP), 25.12.2021 06:50



History, 25.12.2021 06:50

Social Studies, 25.12.2021 06:50

Social Studies, 25.12.2021 06:50

 .
. .
. m/s
m/s