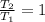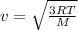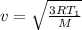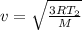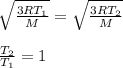Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 21:30, tashaylinm02
Which sections of the heating curve illustrate this process?
Answers: 2


Physics, 23.06.2019 05:00, maljoh8249
Consider the phase change taking place in the picture. you could use all but one statement to describe what is happening here. that is: a) temperature is increasing. b) kinetic energy is increasing. c) the space between the particles remains the same. d) the attractive force between the particles is decreasing.
Answers: 1
You know the right answer?
It is found that the most probable speed of molecules in a gas at equilibrium temperature
T2 is the...
Questions in other subjects:





History, 24.09.2020 21:01



Mathematics, 24.09.2020 21:01

Social Studies, 24.09.2020 21:01