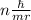Physics, 12.05.2021 01:00 payshencec21
The key insight that Bohr introduced to his model of the atom was that the angular momentum of the electron orbiting the nucleus was quantized. He introduced the postulate that the angular momentum could only come in quantities of nh/(2π), where h is Planck's constant and n is a nonnegative integer (0,1,2,3,…). Given this postulate, what are the allowable values for the velocity v of the electron in the Bohr atom? Recall that, in circular motion, angular momentum is given by the formula L= mvr.

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 11:00, Arielledt10
1. jay fills a wagon with sand (about 20 kg) and pulls it with a rope 30 m along the beach. he holds the rope 25° above the horizontal. the rope exerts a 20-n tension force on the wagon. how much work does the rope do on the wagon?
Answers: 1

Physics, 23.06.2019 00:20, bighoneypadrick
An object with a mass of 1.5 kg changes its velocity from + 15 m/s to +22 m/s during a time interval of 3.5 seconds. what impulse was delivered to the objects?
Answers: 2

Physics, 23.06.2019 00:50, lbelle
Aplayer uses a hockey stick to push a puck at a constant velocity across the ice. the weight of the puck is 170 n. the coefficient of friction is 0.0600. with what force must the player push the puck so that his force just equals the frictional force? 28.3 n 0.999 n 0.102 n 0.0104 n
Answers: 1

Physics, 23.06.2019 10:00, meganwintergirl
Two point charges, separated by 1.5cm, have charge values of +2.0 and-4.0 uc, respectively. what is the value of the mutual force between them
Answers: 3
You know the right answer?
The key insight that Bohr introduced to his model of the atom was that the angular momentum of the e...
Questions in other subjects:


English, 18.02.2021 21:40

Mathematics, 18.02.2021 21:40

Social Studies, 18.02.2021 21:40

History, 18.02.2021 21:40

Mathematics, 18.02.2021 21:40