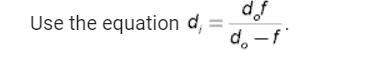Physics, 07.05.2021 01:00 jordynnmarelich1194
A system consisting of 0.0387 moles of a diatomic ideal gas is taken from state A to state C along the path in the figure below. Point A (2 L, 3 atm) --> point B (2 L, 8 atm) --> point C (8 L, 6 atm).
(A) How much work is done on the gas during this process?
(B) What is the lowest temperature of the gas during this process?
(C) Find the change in internal energy of the gas in going from A to C. Hint: Adapt the equation (for the change in internal energy of a monatomic ideal gas)
(D) (d) Find the energy delivered to the gas in going from A to C.

Answers: 3


Other questions on the subject: Physics

Physics, 21.06.2019 15:30, lazarovalle3598
Which of the following statements are true of solids? a. the particles do not vibrate. b. the particles are in a fixed location. c. they have strong intermolecular forces between the atoms or molecules. d. the particles have less kinetic energy than those of liquids or gases.
Answers: 1

Physics, 21.06.2019 22:10, sanders8151
How would doubling the made of an object change the objects potential energy
Answers: 2

Physics, 22.06.2019 09:40, wi8wuwj283jendjdudjd
When you jump from an elevated position you usually bend your knees upon reaching the ground. by doing this, you make the time of the impact about 10 times as great as for a stiff-legged landing. in this way the average force your body experiences is a) less than 1/10 as great. b) more than 1/10 as great. c) about 1/10 as great. d) about 10 times as great.
Answers: 1
You know the right answer?
A system consisting of 0.0387 moles of a diatomic ideal gas is taken from state A to state C along t...
Questions in other subjects:

English, 14.07.2019 07:30








History, 14.07.2019 07:30