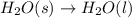During melting, the forces of attraction between the molecules
a) become weaker.
b) rem...


Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 00:30, Solany6426
Part f - example: finding two forces (part i) two dimensional dynamics often involves solving for two unknown quantities in two separate equations describing the total force. the block in (figure 1) has a mass m=10kg and is being pulled by a force f on a table with coefficient of static friction îľs=0.3. four forces act on it: the applied force f (directed î¸=30â above the horizontal). the force of gravity fg=mg (directly down, where g=9.8m/s2). the normal force n (directly up). the force of static friction fs (directly left, opposing any potential motion). if we want to find the size of the force necessary to just barely overcome static friction (in which case fs=îľsn), we use the condition that the sum of the forces in both directions must be 0. using some basic trigonometry, we can write this condition out for the forces in both the horizontal and vertical directions, respectively, as: fcosî¸â’îľsn=0 fsinî¸+nâ’mg=0 in order to find the magnitude of force f, we have to solve a system of two equations with both f and the normal force n unknown. use the methods we have learned to find an expression for f in terms of m, g, î¸, and îľs (no n).
Answers: 2


Physics, 22.06.2019 08:50, dijonmckenzie3
The electronic structure or chlorine is 2.8.7 what is the electronic structure of fluorine?
Answers: 2

Physics, 22.06.2019 10:00, amyaacrawford86
Because air contracts as it cools, the air pressure inside a freezer is typically lower than on the outside. why do ice cubes inside a freezer tend to shrink over time? a. the ice dissolves oxygen from the air, forming a denser crystalline matrix. b. the ice reacts chemically with carbon dioxide in the air, forming gaseous carbon compounds. c. the ice melts, and then the liquid freezes as ice crystals on the bottom of the freezer. d. the ice sublimes, and then the water vapor deposits as ice crystals on the sides of the freezer.
Answers: 1
You know the right answer?
Questions in other subjects:






Biology, 05.05.2020 09:11




Biology, 05.05.2020 09:11