

Answers: 2


Other questions on the subject: Physics


Physics, 22.06.2019 09:30, officialariana01
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 3

Physics, 22.06.2019 10:30, legendman27
You are given two vectors a⃗ =−3.00ι^ 5.00j^ and b⃗ =5.00ι^ 2.00j^. let the counterclockwise angles be positive.
Answers: 3

Physics, 22.06.2019 11:30, genyjoannerubiera
A100-watt light bulb illuminates a solar cell. the electricity from the solar cell operates a water pump that delivers 1 watt of power. what is the efficiency of the system?
Answers: 2
You know the right answer?
Which equation best expresses the first law of thermodynamics, assuming q is heat, u is internal ene...
Questions in other subjects:


Physics, 29.03.2021 14:00


English, 29.03.2021 14:00



History, 29.03.2021 14:00

French, 29.03.2021 14:00

Mathematics, 29.03.2021 14:00

Mathematics, 29.03.2021 14:00

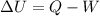 ''
'' is the change in internal energy
is the change in internal energy 

