
How does the law of conservation of mass apply to this reaction: mg + hcl → h2 + mgcl2?
the equation needs to be balanced. there are fewer hydrogen atoms in the equation than magnesium or chlorine.
only the hydrogen needs to be balanced. there are equal numbers of magnesium and chlorine.
the law of conservation of mass has already been applied. there is an equal number of each element on both sides of the equation.
hydrogen and chlorine need to be balanced. there is an equal amount of magnesium on each side

Answers: 1


Other questions on the subject: Physics


Physics, 22.06.2019 17:00, davinedmcneal
If you wanted to move an electron from the positive to the negative terminal of the battery, how much work w would you need to do on the electron? enter your answer numerically in joules.
Answers: 1


Physics, 23.06.2019 11:00, selenaK9514
A40 kilogram skier starts at the top of a 12 meter heigh slope. at the bottom she is traveling 10 meters per second how much does she lose to friction? a. 4,704 joules b. 2,704 joules. c.2,000 joules d. she doesn’t lose any because mechanical energy is conserved. 35
Answers: 1
You know the right answer?
How does the law of conservation of mass apply to this reaction: mg + hcl → h2 + mgcl2?
Questions in other subjects:


Mathematics, 05.05.2020 15:07

English, 05.05.2020 15:07





Mathematics, 05.05.2020 15:07

Chemistry, 05.05.2020 15:07

History, 05.05.2020 15:07

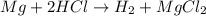
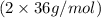 = 96 g/mol
= 96 g/mol![(24 g/mol + [2 \times 35 g/mol])](/tpl/images/0498/6834/34391.png) = 96 g/mol
= 96 g/mol

