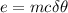Physics, 18.04.2021 14:30 keiolanibrooks
After the water has boiled, the temperature of the water decreases by 22 °C.
The mass of water in the kettle is 0.50 kg.
The specific heat capacity of water is 4200 J/kg °C.
Calculate the energy transferred to the surroundings from the
water.
Energy=

Answers: 1


Other questions on the subject: Physics


Physics, 22.06.2019 12:00, Emieloy2959
Ihave a density of 1.61g/cm^3 and a mass of 28g. find the missing value
Answers: 1

Physics, 22.06.2019 13:00, chie42
Which of the following correctly describes what happens when an atomic bomb explodes? small pieces of fissionable material are joined and form a body with a mass greater than the critical mass, the relative number of neutrons escaping decreases, and a chain reaction and explosion result. large pieces of fissionable matter are brought together quickly and form a body with a mass smaller than the critical mass, the relative number of escaping neutrons increases, and a chain reaction and explosion result.
Answers: 2

Physics, 22.06.2019 14:20, mercymain1014
How many atoms of nitrogen are in the chemical formula ni(w on
Answers: 1
You know the right answer?
After the water has boiled, the temperature of the water decreases by 22 °C.
The mass of water in t...
Questions in other subjects:




History, 21.01.2022 14:00


History, 21.01.2022 14:00

History, 21.01.2022 14:00

Chemistry, 21.01.2022 14:00

Arts, 21.01.2022 14:00

Chemistry, 21.01.2022 14:00