
Physics, 09.03.2021 18:20 ilvvsShatalov3984
A student is studying the ways different elements are similar to one another. Diagrams of atoms from four different elements are shown below. Which two atoms are of elements in the same group in the periodic table?
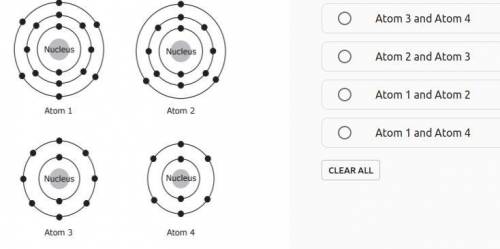

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 12:30, jaejaeJae941
Urgent pls a. coal consumption levels off and remains flat. b. petroleum, natural gas, and renewables show an increase in consumption c. more nonrenewable resources continued to be consumed than renewable. d. there is little projected increase in nuclear energy use. e. carbon dioxide emissions are projected to decline as we approach 2040. global energy consumption is defined as the total energy used by an individual or organizations from around the world. use the graph above to analyze the projected energy consumption from now until 2040. which statements in the prompt apply? a) a, b, d b) b, c, d c) a, c, d d) a, b, c, d
Answers: 1



Physics, 23.06.2019 03:20, marshaehayes9444
Neutrons are placed in a magnetic field with magnitude 2.30 t. part a part complete what is the energy difference between the states with the nuclear spin angular momentum components parallel and antiparallel to the field? δe δ e = 2.77×10−7 ev previous answers correct part b part complete which state is lower in energy: the one with its spin component parallel to the field or the one with its spin component antiparallel to the field? which state is lower in energy: the one with its spin component parallel to the field or the one with its spin component antiparallel to the field? parallel antiparallel previous answers correct part c part complete how do your results compare with the energy states for a proton in the same field (δe=4.05×10−7ev)? how do your results compare with the energy states for a proton in the same field this result is smaller than but comparable to that found in the example for protons. this result is greater than but comparable to that found in the example for protons. previous answers correct part d the neutrons can make transitions from one of these states to the other by emitting or absorbing a photon with energy equal to the energy difference of the two states. find the frequency of such a photon. f f = mhz previous answersrequest answer incorrect; try again; 5 attempts remaining
Answers: 2
You know the right answer?
A student is studying the ways different elements are similar to one another. Diagrams of atoms from...
Questions in other subjects:

Mathematics, 25.04.2020 16:27




Business, 25.04.2020 16:28



Mathematics, 25.04.2020 16:29

Business, 25.04.2020 16:29



