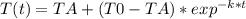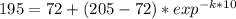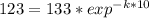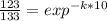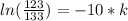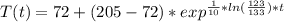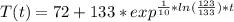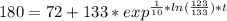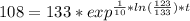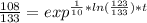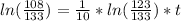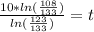You place a cup of 205of coffee on a table in a room that is 72of, and 10 minutes later, it is 195of. approximately how long will it be before the coffee is 180of? use newton's law of cooling: \[t(t)=t _{a}+(t _{o}-t _{a})e ^{-kt}\]
a. 15 minutes
b. 25 minutes
c. 1 hour
d. 45 minutes

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 03:30, nkazmirski5598
Aplane flies a distance d from west to east at a constant speed vp , with respect to the air in a time twe . on the return trip, the plane flies a distance d from east to west at a constant speed vp , with respect to the air in a time tew . on both trips the wind blows from west to east at a constant speed va , with respect to the ground 1) what is tew in terms of vp, va, and d, as needed? tew=dvp tew=dvp+va tew=dva tew=dvpâ’va tew=12dvp tew=12dvp+va tew=12dvpâ’va your submissions: b submitted: saturday, january 26 at 6: 49 pm feedback: feedback will be given once all questions have been attempted and the grade cluster button has been pressed. 2) what is twe in terms of vp, va, and d, as needed? twe=dvp twe=dvp+va twe=dva twe=dvpâ’va twe=12dvp twe=12dvp+va twe=12dvpâ’va your submissions: d submitted: saturday, january 26 at 6: 49 pm feedback: feedback will be given once all questions have been attempted and the grade cluster button has been pressed. 3) assuming d = 2300 miles, vp = 400 miles/hr, and twe = 4 hours, what is va, the speed of the wind with respect to the ground? va = 100 miles/hr va = 575 miles/hr va = 200 miles/hr va = 175 miles/hr va = 975 miles/hr 4) once again, assuming d = 2300 miles, vp = 400 miles/hr, and twe = 4 hours, what is tew, the time it takes the plane to fly a distance d miles from east to west? tew = 5.75 hours tew = 11.5 hours tew = 7.67 hours tew = 8 hours tew = 10.2 hours
Answers: 2


Physics, 23.06.2019 10:40, puzzledprincess8037
4.at 0 ºc, some amount of energy is required to change 1 kg of water from a solid into a liquid. if you had a 2 kg piece of ice, what effect would this have on the amount of thermal energy required to change the water from a solid to a liquid? it would require more energy to change solid water into liquid water because there are more molecules in this larger piece of ice. it would still require the same amount of energy to change solid water into liquid water because the entire piece of ice would still gain the same amount of energy in each case. it would require energy to be removed from the 2 kg piece of ice. the larger piece of ice already has more total energy than the smaller piece of ice, so energy must be removed for the ice to become liquid. it would require less energy to change solid water into liquid water because the energy would spread through the ice more quickly and the ice already has a larger total amount of energy because it is larger than a 1 kg piece of ice.
Answers: 1

Physics, 23.06.2019 11:00, tatiiiee
Abox that has self-contained cable clamps holds two light switches. a two-wire no. 12 cable with ground feeds the box, and a pair of two-wire no. 12 cables (four conductors) with ground wires leaves the box to feed the light fixtures. the second switch is fed by a no. 12 jumper wire from the first switch. which one of the following box combinations would furnish the minimum allowable volume? a. two 3-inch × 2-inch × 21⁄2-inch gangable device boxes b. two 3-inch × 2-inch × 11⁄2-inch gangable device boxes c. two 3-inch × 2-inch × 23⁄4-inch gangable device boxes d. two 3-inch × 2-inch × 21⁄4-inch gangable device boxes
Answers: 2
You know the right answer?
You place a cup of 205of coffee on a table in a room that is 72of, and 10 minutes later, it is 195of...
Questions in other subjects:

Mathematics, 29.01.2021 19:30




Mathematics, 29.01.2021 19:30

English, 29.01.2021 19:30


Mathematics, 29.01.2021 19:30


Mathematics, 29.01.2021 19:30